How sleep loss sabotages new memory storage in the hippocampus
While some students may think it’s a good idea to pull an all-nighter before an exam, conventional wisdom may be correct: a good night’s sleep may actually be more helpful, according to University of Michigan research.
U-M scientists Sara Aton and James Delorme found when mice are sleep deprived, there is an increase in activity in inhibitory neurons in the hippocampus, an area of the brain essential for navigation, as well as for processing and storing new memories.
“Because these neurons limit activity in their neighbors, this physiological response makes it impossible to muster normal neuronal activity in the hippocampal structure,” said Sara Aton, an associate professor in the U-M Department of Molecular, Cellular and Developmental Biology and a member of the U-M Center for RNA Biomedicine executive committee. “I always tell my students that an overnighter is not helping them prepare for an exam.”
The researchers’ results are published in the Proceedings of the National Academy of Sciences, and their findings could have implications for human performance and learning strategies.
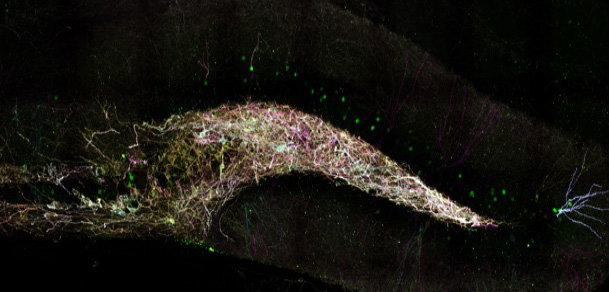
Image: Somatostatin-expressing interneurons in the mouse dentate gyrus, labeled with Brainbow 3.0 (which labels each neuron a distinct color). cFos (labeled green) is present in the nuclei of surrounding pyramidal cells which are active during sleep. Image by Frank Raven
Previous research has shown there is a sensitive window of time—a few hours following learning—during which mice have to sleep in order to fully consolidate a memory. During this period, neuronal activity must remain undisturbed in the hippocampus, and RNA transcription and translation within the neurons must occur normally. Aton and Delorme, formerly a U-M neuroscience graduate student, studied the possible links between changes in neurons’ activity after learning and changes in their protein translation.
First, Delorme investigated the interaction between sleeping and waking, hippocampal neuron activity, and activity-driven phosphorylation of S6, a component of ribosomes—tiny organelles which translate mRNA into protein. This phosphorylation event is thought to affect which mRNAs that are being translated into protein as neurons become more active. This regulation may be important for adapting to neurons’ ever-changing metabolic demands.
To do this, he gave mice a fear stimulus. When mice were allowed to freely sleep following the stimulus, Delorme saw that S6 phosphorylation increased in a part of the hippocampus called dentate gyrus, the first region where memories begin to form. But when the mice were deprived of sleep, Delorme found that phosphorylation decreased throughout the hippocampus. This disrupted the mice’s memories that otherwise would have been formed in response to the fear stimulus.
Delorme’s next question was whether this reduction in activity-driven S6 phosphorylation affected all neurons similarly after sleep loss. Using bioinformatics, he compared the abundance mRNAs associated with phosphorylated S6-containing ribosomes. He also examined mRNA profiles under conditions of prior sleep or no sleep.
Delorme then collaborated with U-M Advanced Genomics core for the RNA sequencing. He observed that, after sleep deprivation, there was a significant increase in abundance of a type of RNA transcripts known to be present specifically in interneurons that express the neuropeptide somatostatin as well as the inhibitory neurotransmitter GABA. This relative increase suggested that greater activity among somatostatin-containing interneurons inhibits surrounding neurons, and thus overall S6 phosphorylation in the hippocampus, acting as a gate that slows down their firing.
When they mimicked this inhibitory gating mechanism in freely sleeping mice, they were able to disrupt hippocampal activity and memory consolidation. In contrast, suppressing the activity of somatostatin-expressing interneurons after learning increased activity among dentate gyrus neurons, and was beneficial to memory consolidation.
In disorders such as Alzheimer’s disease, where sleep difficulties are common, there could be a relation between the physiological mechanism described in this study and memory loss. Another implication from these results is that sleep loss could at times be therapeutic – for example, sleep deprivation could be used after a traumatic event to prevent post traumatic stress syndrome.
This study opens the door to further investigate how manipulating the relative balance between the activity of excitatory and inhibitory neurons affects memory, as well as comparing how these mechanisms are affected between REM and non-REM sleep.
Study:
Sleep loss drives acetylcholine- and somatostatin interneuron-mediated gating of hippocampal activity, to inhibit memory consolidation, James Delorme1, Lijing Wang, Femke Roig Kuhn, Varna Kodoth, Jingqun Ma , Jessy D. Martinez, Frank Raven, Brandon A. Toth, Vinodh Balendran, Alexis Vega Medina, Sha Jiang, Sara J. Aton, PNAS, 10.1073/pnas.2019318118

