Thank you for joining us on Friday for Connecting Voices in RNA Care, a patient advocacy panel happening as part of the 2025 RNA Symposium.
The panel featured Michigan Representative Jason Morgan, a rare disease advocate and author of the bill that created the Michigan Rare Disease Advisory Council (MI-RDAC), and other members of the genetic and rare disease communities. The panel was moderated by Dr. Peter Todd and Dr. Beth Ames.
More on this story coming soon!

9th Annual RNA Symposium: From Sequence to Solutions
March 6-8, 2025 | Kahn Auditorium, University of Michigan, Ann Arbor Campus
Register to attend the symposium virtually!
Virtual registration is available now – click the button below.
On-site registration will also be available at 12:00 p.m. Thursday; 8:00 a.m. Friday and Saturday —
one hour before scheduled programming begins each day
Register at the check-in desk in the BSRB Atritum on Thursday and Friday, and the Palmer Commons Atrium on Saturday.
PLEASE NOTE: You will be notified that you must have an Iris account in order to register.
- Visit the Registration Page in the link at the button above
- On the sign-in screen, sign in with your Iris account credentials or select “Don’t have an account, Sign Up”
- Follow the instructions to create your account and register for the Symposium
- Creating your account will allow you to log back in to edit your registration responses
Welcome to our sponsors!
The Center for RNA Biomedicine at the University of Michigan, in partnership with the Society for RNA Therapeutics, proudly invites you to the 2025 RNA Symposium. Taking place from Thursday, March 6 to Saturday, March 8, 2025, this premier event will convene thought leaders and pioneering researchers in the field of RNA science and biomedicine.
2025 RNA Symposium speakers:

John Cooke, M.D., Ph.D.
Joseph C. “Rusty” Walter and Carole Walter Looke Presidential Distinguished Chair in Cardiovascular Disease Research,
Department of Cardiovascular Sciences
Director,
Center for Cardiovascular Regeneration
Medical Director,
Center for RNA Therapeutics
Houston Methodist Hospital
“An academic network for design, development, and delivery of personalized mRNA cancer vaccines”
Abstract:
We have established a core facility that possesses the infrastructure that comprises the technologies, manufacturing, regulatory oversight, and centralized reporting to allow for the safe and effective implementation of personalized mRNA cancer vaccines. Currently the core facility holds an investigational new drug approval from the FDA for a personalized mRNA cancer vaccine program in the treatment of triple negative breast cancer. While this core structure will initially focus on triple negative breast cancer, with success of the strategy, we intend to build a network of outlying hospitals, and a self-sustainable pipeline to deliver new vaccines for any cancer which are accessible and affordable.

Beverly L. Davidson, Ph.D.
Katherine A. High Chair in Cell and Gene Therapy
Director, Raymond G. Perelman Center for Cellular and Molecular Therapeutics
Chief Scientific Strategy Officer
Children’s Hospital of Philadelphia
Professor of Pathology and Laboratory Medicine
Perelman School of Medicine at the University of Pennsylvania
“Engineering newer generations of AAVs for brain delivery and therapy”
Abstract:
Management of neurodegenerative diseases can benefit from brain delivery of therapeutically relevant proteins including recombinant antibodies and their derivatives, growth factors, or pro-enzymes delivered either indirectly from intravenous injection or directly by infusion into the cerebral spinal fluid (CSF). These include adult and childhood onset disorders such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS) and lysosomal storage disorders (LSDs). Gene therapy provides an alternative method for sustained expression of therapeutic proteins. Indeed, recent work shows that prolonged expression of recombinant proteins after adeno-associated virus (AAV) delivery to long-lived, ventricle-lining ependymal cells can profoundly impact disease phenotypes in AD mice and LSD dogs. To advance this work to human application, we embarked on a capsid discovery project using a large library of AAV variants screened through relevant models, with the goal of identifying a capsid with improved therapeutic utility. Through this work, we discovered an AAV capsid with unprecedented potency in transducing ependymal cells and cerebral neurons in NHPs. The identified capsid’s potency was conserved in three species of NHP, two mouse strains, and human neurons derived from induced pluripotent stem cells (iPSCs). Importantly, this capsid provided for robust and therapeutically relevant protein expression in NHPs and mice after CSF delivery at doses well below those currently used in the clinic, showing efficacy delivering two different types of payloads for treatment of recessive lysosomal storage diseases or a genetic form of ALS.

Adrian R. Krainer, Ph.D.
Professor
St. Giles Foundation Professor
Cancer Center Program Co-Leader
Cold Spring Harbor Laboratory
“Antisense therapy for H3.3K27M-related diffuse midline glioma”
Abstract:
Diffuse midline gliomas (DMGs) are pediatric high-grade brain tumors in the thalamus, midbrain, or pons; the latter are called diffuse intrinsic pontine gliomas (DIPG) or H3.3K27M-related DMG. The brain-stem location limits the clinical management of these tumors, resulting in exceedingly poor outcomes. A heterozygous point mutation in one of two non-canonical histone H3.3 genes is present in most DIPG tumors. This dominant, somatic mutation alters H3-3A, replacing lysine 27 with methionine (K27M) in the protein, and results in global reduction of K27 tri-methylation of all wild-type histone H3 proteins, which is a driving event in gliomagenesis. We developed a lead antisense oligonucleotide (ASO) that directs RNase-H-mediated knockdown of H3-3A mRNA. ASO treatment restored K27 trimethylation of histone H3 proteins and significantly reduced tumor growth, promoted neural-stem-cell differentiation, and increased survival in two DIPG mouse models. As an alternative strategy to reduce expression of the toxic mutant protein, we are developing a lead splice-switching ASO that forces skipping of the exon encoding the mutation and the start codon. Our results demonstrate the involvement of the H3.3K27M oncohistone in tumor maintenance, and the reversibility of the aberrant epigenetic changes it promotes, in addition to providing preclinical proof-of-concept for DMG antisense therapy.

Mats Ljungman, Ph.D.
Professor,
Radiation Oncology
Environmental Health Science
Co-Director,
Center for RNA Biomedicine
University of Michigan
“Targeting cancer with personalized precision RNA therapeutics”
Abstract:
Current anti-cancer treatments give rise to dose-limiting toxicity in normal tissues. We have developed a precision CRISPR-based approach to target genomic features that are unique to cancer cells. This approach, KLIPP, utilizes a “split-enzyme” dead Cas9 fused to an endonuclease Fok1 where two Fok1-dCas9 complexes need to homodimerize to activate the endonuclease. Using whole genome sequencing of the patient’s cancer, we identify cancer-specific structural variant junctions (SVJs) and design sgRNA that binds to sequences flanking the SVJs. We show that KLIPP, delivered using lipid nanoparticles, induces DNA double strand breaks specifically in cancer cells causing toxicity. SVJs in fusion oncogenes and in amplicons of oncogenes, such as on ecDNA, are of specific interest to target with KLIPP.

Muthiah (Mano) Manoharan, Ph.D.
Senior Vice President of Drug Innovation,
Scientific Advisory Board Member,
Distinguished Research Scientist
Alnylam Pharmaceuticals
“Biomimetic chemistry of RNA therapeutics”
Abstract:
According to Professor Ron Breslow, “biomimetic chemistry” is new chemistry inspired by the principles used by Nature. Synthetic small interfering RNAs (siRNAs) are potent inhibitors of gene expression. These molecules are perfect examples of biomimetic chemistry as synthetic siRNAs act through the natural RNA interference (RNAi) pathway. To deliver therapeutic siRNAs into human liver, we developed approaches that include chemical modification of the siRNAs and either lipid nanoparticle (LNP) formulation or multivalent N-acetylgalactosamine (GalNAc) conjugation, making possible intravenous and subcutaneous administration, respectively. The design of chemical modifications of siRNAs to enable favorable Argonaute2 (Ago2) recognition as well as both delivery strategies rely on biomimetics. The LNP approach is based on the endogenous Apo-E ligand /LDL receptor process. Conjugation of the GalNAc ligand to an siRNA mediates it uptake into liver hepatocytes through the asialoglycoprotein receptor. Using these strategies five approved RNAi therapeutics have emerged from Alnylam. We have also used lipid conjugates for CNS delivery of therapeutic siRNAs. This presentation will cover the molecular basis of RNA therapeutics including the chemical modifications and motifs used in each RNA strand to ensure uptake into cells of the targeted tissue, Ago2 recognition, silencing efficiency, metabolic stability, and safety.

Anna Marie Pyle, Ph.D.
Sterling Professor,
Yale University
Molecular, Cellular and Developmental Biology
Chemistry,
Howard Hughes Medical Institute
“Panning for gold: Discovering and drugging riboregulatory structures within viral genomes and eukaryotic mRNAs”
Abstract:
It is becoming increasingly clear that RNA viruses have highly structured RNA genomes, even within ORF regions, and that many the of these folded structural motifs play essential roles in viral life cycles. Moreover, riboregulatory strategies discovered in viral genomes are often subsequently identified within our own mRNAs, making it all the more important to mine viral genomes for mechanistic information. Despite our increasing awareness of riboregulation in many viral families, some of the most interesting genomes have remained structurally and functionally uncharacterized. Here we describe a pipeline for rapid discovery, structural and functional characterization of critical riboregulatory motifs within the genomes of novel RNA virus families, with a special focus on flaviviruses and noroviruses, and a particular emphasis on complex structural motifs within ORFs. This type of analysis not only charts the landscape of RNA structures and riboregulatory elements, it provides a new means for identifying therapeutic targets within pathogens and within human genes themselves.
Join us for a series of dynamic presentations, panel discussions, and collaborative sessions designed to push the boundaries of RNA research and foster interdisciplinary collaboration.
Dates: March 6 – 8, 2025
Location: University of Michigan Ann Arbor Campus
Symposium and Gala Dinner Registration Fees
Symposium Registration Fees
| Registration type | Early bird (Oct-Dec 31) | Regular (After Dec 31) |
| U-M student/postdoc | $25 | $30 |
| Non U-M student/postdoc | $60 | $72 |
| U-M academic/nonprofit | $60 | $72 |
| Non U-M academic/nonprofit | $120 | $144 |
| Industry/corporate | $250 | $300 |
| Virtual/streaming Enjoy the programming from the comfort of your own device (please note: sessions will not be recorded) | $30 | $36 |
Gala dinner info and pricing
All attendees are invited to join us for a gala dinner on the first evening of the Symposium. Network with speakers, presenters, and fellow attendees in a relaxed and enjoyable setting. Reserve your seat now for a memorable evening of connections and inspiration!
| Registration Type | Early bird (Oct-Dec 31) | Regular (After Dec 31) |
| Student/ postdoc | $25 | $30 |
| Faculty/ staff | $50 | $60 |
| Industry/ corporate | $75 | $90 |
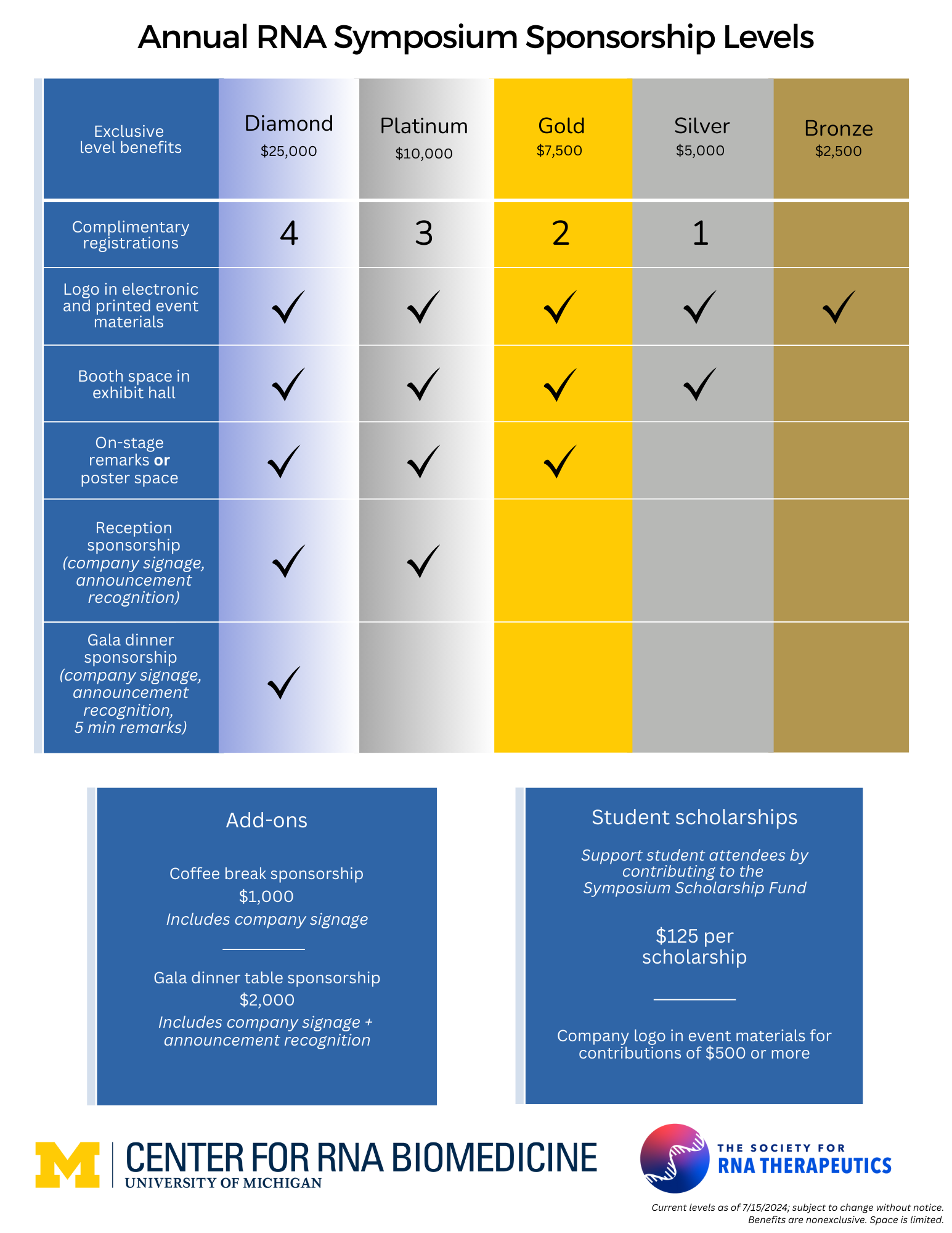
Sponsorship Information
Seize this unparalleled opportunity to drive groundbreaking science by becoming a sponsor for the 9th Annual RNA Symposium. For the first time, this event will be in collaboration with the University of Michigan’s Center for RNA Biomedicine and the Society for RNA Therapeutics.
This is not just another symposium—it’s the beginning of an exciting new era in RNA research!
Questions? Contact RNA Program Manager, Maria Stieve at mstieve@umich.edu
Click the button below to view a detailed program of the 9th Annual RNA Symposium: From Sequence to Solutions on our Symposium Hub










